Surplus ammunition has its risks. This stuff was examined by ammunition specialists in the country of origin, determined to be either unsafe to issue or unsafe to store, and it was removed from inventory.
Then it is sold to American shooters who have been kept ignorant of the aging processes that occurr in old ammunition.
Ammunition has a shelf life. The lifetime varies, primarily on storage temperature, but as ammunition gets older, it does not get better.
From what I had read on the internet, which is a repeat of what is said in gun magazines, powder has an “indefinite” shelf life. Remember reading statements to the effect that powder lost energy as it got old, making it essentially benign. The first part is true, the second part is not. Luckily, I ran into an Insensitive Munitions expert. This IM expert explained that powder deteriorates from the day it leaves the factory.
Nitrocellulose decomposes through the reduction-oxidation process. Called Redox. The expert said “The molecular stability of the functional groups on the organic chain determine the life time of the nitrocellulose molecule.” All ionic compounds, water is the main offender because it is always in air, react with those bonds and accelerates the deterioration of the powder.
The bottom line is that nitrocellulose is a high energy molecule that wants to become a low energy molecule. Gunpowder like all other substances follows the laws of nature.
Heat accelerates the deterioration/decomposition of powder and the rate is an exponential function. Small increases in temperature result in faster and faster deterioration of the gunpowder. Powder that could have a storage lifetime of decades at 70 F will last weeks at 150 F. If you read in the Insensitive munitions literature, you will see that they use high temperature to accelerate aging of smokeless propellants.
ROLE OF DIPHENYLAMINE AS A STABILIZER IN PROPELLANTS;
ANALYTICAL CHEMISTRY OF DIPHENYLAMINE IN PROPELLANTS
Nitrocellulose-base propellants are essentially unstable materials that decompose on aging with the evolution of oxides of nitrogen. The decomposition is autocatalytic and can lead to failure of the ammunition or disastrous explosions.
http://www.dtic.mil/dtic/tr/fulltext/u2/783499.pdf
Heat, as you can see in this report, will age gunpowder
Combustion pressures will rise after high temperature storage.
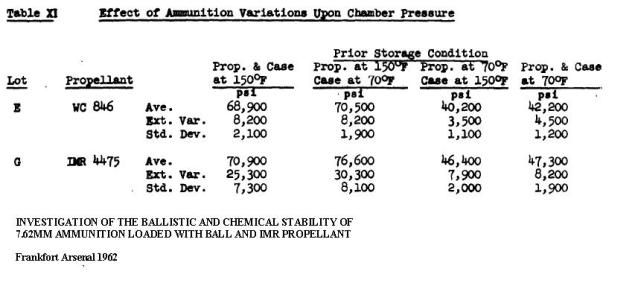
INVESTIGATION OF THE BALLISTIC AND CHEMICAL STABILITY OF 7.62MM AMMUNITION LOADED WITH BALL AND IMR PROPELLANT
Frankfort Arsenal 1962
3. Effects of Accelerated Storage Propellant and Primer Performance
To determine the effect of accelerated isothermal storage upon propellant and primer performance, sixty cartridges from each of lots E (WC 846) and G (R 1475) were removed from 150F storage after 26 and 42 weeks, respectively. The bullets were then removed from half the cartridges of each lot and from an equal number of each lot previously stored at 70F. The propellants were then interchanged, the bullets re-inserted, and the cases recrimped. Thus, four variations of stored components were obtained with each lot.
Chamber pressures yielded by ammunition incorporating these four variations were as follows. These values represent averages of 20 firings.
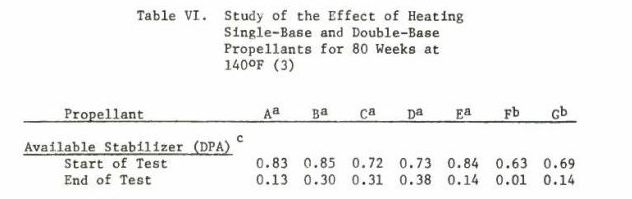
Double based powders have a reduced lifetime compared with single base. Double based powders have nitroglycerin (NG) in the grain. Nitroglycerine remains a liquid and it migrates within the grain to react with the NO bonds on the nitrocellulose, increasing the rate of reduction-oxidation reaction. All ionic compounds react with those bonds and accelerate the deterioration of the powder. Rust is bad as ferric oxide is ionic. Water is polar covalent ion and is ever present in the air.
Because water reacts in a negative way with smokeless propellants, quality ammunition is manufactured in humidity controlled environments. Between 40% and 20% humidity. They don't go lower due to electro static discharge concerns.
The best storage condition for powders is arctic. Cold and dry.
Due to the migration of NG within double based powders, the surface of the grain will become rich in NG even though the total energy content of the propellant has decreased. This will cause changes in the burn rate, and can cause pressures to spike. The surface of nitrocellulose powders also change as the powder deteriorates, and it changes unevenly. This creates conditions for erratic burn rates. Burn rate instability is undesirable and can cause explosive conditions in firearms. In retrospect, this explains the “funny” retorts I experienced and the sticking cases. It is an extremely rare occurrence, but old ammunition has caused rifle Kabooms. When I discussed this with a machine gunner buddy, he said that explained the two top cover explosions he had with old Yugoslavian 8 MM ammo. I think it explains the Garand kaboom in the link below.
Section from the Propellant Management Guide:
Stabilizers are chemical ingredients added to propellant at time of manufacture to
decrease the rate of propellant degradation and reduce the probability of auto ignition during its expected useful life.
As nitrocellulose-based propellants decompose, they release nitrogen oxides. If the nitrogen oxides are left free to react in the propellant, they can react with the nitrate ester, causing further decomposition and additional release of nitrogen oxides. The reaction between the nitrate ester and the nitrogen oxides is exothermic (i.e., the reaction produces heat). Heat increases the rate of propellant decomposition. More importantly, the exothermic nature of the reaction creates a problem if sufficient heat is generated to initiate combustion. Chemical additives, referred to as stabilizers, are added to propellant formulations to react with free nitrogen oxides to prevent their attack on the nitrate esters in the propellant. The stabilizers are scavengers that act rather like sponges, and once they become “saturated” they are no longer able to remove nitrogen oxides from the propellant. Self-heating of the propellant can occur unabated at the “saturation” point without the ameliorating effect of the stabilizer. Once begun, the self-heating may become sufficient to cause auto ignition.
NOx gas is a mix of compounds all of which are reactive.
http://en.wikipedia.org/wiki/NOx http://en.wikipedia.org/wiki/Nitrogen_oxide When smokeless propellants break down NOx gas is released. Nitric acid gas is only produced in the presence of water, because it requires a hydronimun ion, but there is plenty of water in air.
The Armed Forces have stockpile surveillance programs but each Service does theirs a little differently. If you want to see all the different tests the military uses to determine propellant characteristics, look at Mils Std 286 Propellants, Solid: Sampling, Examination and Testing to be found at
https://assist.daps.dla.mil/quicksearch/.
If you look, you will find aging tests. One common test is for powder to be kept at 65 C (150 F) until it fumes. It if fumes within 30 days it is checked for stabilizer or scrapped.
The Navy expert told me a few ways the Navy samples its powders and propellants. If the powder is outgassing nitric gas (as determined by change of color of methly violet paper in contact with the powder (Methly Violet test, or Talliani test)), the stuff is tested to see how much stabilizer is left. If the amount is less than or equal to 20%, the lot is scrapped.
Scrapping powders and propellants with this percentage of stabilizer appears to be consistent across all services.
Pages 5-11 of the 2003 Army Logistics Propellant Management Guide provide the protocols for testing and subsequent actions for their Stockpile Propellant Program. Basically, all propellant lots are tracked. The trigger for investigation is: "
When Master Sample Stability Failure Occurs"
The Navy expert provided 'rules of thumb' concerning when to expect problems with double based and single based propellants. The rules of thumb are: Double based powders and ammunition are scrapped at 20 years, single based 45 years. In his words “These 'rules of thumb' are particularly useful when the protocol fails. The protocol can easily fail when workmanship or good housekeeping measures are not followed during manufacture of propellant and/or rocket motor or during storage of the weapon system components, respectively.”
Early in the last century the storage lifetime of smokeless powders was considered to be 20 years or less:
Army Ordnance Magazine, June 1931, page 445 says:
“Smokeless powder constitutes one of the greatest hazards from a storage standpoint, due to the fact that it is subject to deterioration and at the best cannot be expected to have a life greater than about twenty years…….Master samples of all lots of smokeless powder are under constant observation in the laboratories at Picatinny Arsenal. Should any of these samples indicate rapid deterioration, notification is given at once, and steps are taken to use this deteriorating material within a very short period, if possible, or else withdraw it from service.”
For the home reloader, if the powder has turned red, or smells like acid, it is way beyond its safe limits.
I am of the opinion that the reason this is not discussed in the popular gun press is because if the shooting community knew that powders had a shelf life, it might effect sales. As we all know, gunwriters are paid to promote the industry and for decades they have been reassuring us that as powder gets old, it becomes benign. I cannot see a reason why industry wants you, the shooter, to be picky about old powders and old ammunition. You might not buy, you might have reservations about buying. It is all about their profits.
The military does not talk about this, but bunkers and ammunition storage areas have gone Kaboom due to old powder. That nitric acid builds up, creates heat, and the stuff blows up. It blows up inside the case or the shell.
http://www.liveleak.com/view?i=13c_1205681217
This powder is from a FA 11-1898 30-40 Krag cartridge. Obviously it is bad.
I sent the IM expert the link with this Garand blowup,
http://www.socnet.com/showthread.php?p=1344088
and the pulled Krag red powder, and this is what he wrote back:
Wow
The red color indicates that the stabilizer is depleted and the redox reaction is degrading the nitrate ester. (I assume this is a single base gun propellant, and the nitrate ester is NC.) Please dispose of this powder and ammo supply before it starts to get warm or self-heat (via autocatalytic exothermic reaction). This stuff can be a runaway reaction and spotaneously explode in storage.
The cracked case necks are proof that the outgassing of NOx is occurring. The pressure build-up is evidently enough to fatigue the metal at a high stress location in the cartridge case (@ the neck bend). You should also see a bulge in the cartridge base (where the firing pin would strike b/c there is a circular joint crimp there between the two metals). This ammo would explosively vent at the crack if you tried to fire it in a gun. Just like the Garand example you sent. Please discard this ammo.
The corroded ammo is the same as above (redox reaction gassing NOx) except this stuff actually got wet too. Water provides a medium for corrosive acid reactions to result. Please discard this ammo.
Lessons learned -
(1) Ammo has a finite shelf life
(2) Ammo can be dangerous
http://www.usrifleteams.com/forums/index.php?showtopic=21886
Posted 17 July 2012 - 01:29 PM
'Tailgunner', on 17 Jul 2012 - 13:16, said:
I picked up some surplus ammo a couple of years ago and had a couple of hang fires. The hammer would drop and a second later the rifle would discharge.
After that happened a couple of times, I decided I wasn't going to shoot that stuff any more. So I took the ammo apart, thinking I'd at least salvage the brass. After I'd pulled all of the bullets and dumped the powder, I tried chucking the primed cases in a vice and then hit the primers with a pin punch and a hammer.
I found that some of the primers would "pop" but others would just sizzle and smoke. I'm pretty sure those were my hang fires. It was an interesting experiment
The last surplus ammo I had looked so bad that I never fired any of it. Like you, I took it apart. The powder was clumped together. The base of the bullet was green with corrosion. I decapped all the brass, burned the primers and powder outside when burning rubbish, and sold the brass and bullets to a scrap company. Recouped a very small amount of initial price. That was the last time I got fooled on surplus ammo crap.
My guess is that most of the foreign countries that are selling surplus goods to the USA, don't care much about how they handle or store the items, as long as it gets on the shipping container and they pocket the purchase price, they are happy. Caveat emptor is Latin for "Let the buyer beware." It especially applies to surplus goods that have a shelf life. Where was it stored? How was it stored? What temperature? Subjected to water or salt air? Exposed to a structure fire? How was it transported? Etc. etc...too many unanswered questions. A deal that is too good to be true, usually is too good to be true.